CPC Definition - Subclass C08G
This place covers:
Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. condensation polymers, where the polymers are:
Polymers from aldehydes or ketones, the polymers including polyacetals and phenol-formaldehyde-type resins such as novolaks or resoles,
Polymers from isocyanates or isothiocyanates, the polymers including polyurethanes and polyureas,
Epoxy resins,
Polymers obtained by reactions forming a carbon-to-carbon link in the main chain, e.g. Polyphenylenes and polyxylylenes,
Polymers obtained by reactions forming a linkage containing oxygen in the main chain, e.g. Polyesters, polycarbonates, polyethers and copolymers of carbon monoxide with aliphatic unsaturated compounds,
Polymers obtained by reactions forming a linkage containing nitrogen in the main chain, e.g. Polyamides, polyamines, polyhydrazides, polytriazoles, polyimides, polybenzimidazoles and nitroso rubbers,
Polymers obtained by reactions forming a linkage containing sulphur in the main chain, e.g. Polysulphides, polythioethers, polysulphones, polysulphoxides, polythiocarbonates and polythiazoles,
Polymers obtained by reactions forming a linkage containing silicon in the main chain, e.g. Polysiloxanes, silicones or polysilicates,
Other polymers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. Polymers obtained by reactions forming a linkage containing other elements in the main chain, e.g. P, B, AL, Sn, block copolymers obtained by inter-reacting polymers in the absence of monomers, dendrimers and hyperbranched polymers.
Processes for preparing the macromolecular compounds provided for in this subclass.
Subclasses C08B - C08L are generally function-oriented subclasses in relation to the polymers per se, while C09D - C09K are application-oriented subclasses in relation to the said polymers (see below for the special relationship with C09D and C09J).
Polysaccharides per se and their derivatives are classified in C08B.
Treatment and chemical modification of rubbers (homo- or copolymers of dienes classified in C08F 36/00, C08F 136/00, C08F 236/00), are classified in C08C – however synthesis of rubbers and treatment or chemical modification of non-rubbers are classified in subclasses C08F or C08G.
Macromolecular compounds per se obtained by reactions only involving carbon-to-carbon unsaturated bonds (usually known as addition polymers) are in C08F. Compositions based on monomers of such polymers are also in C08F.
Compositions of macromolecular compounds, either with other macromolecular compounds or with other ingredients, including compositions of polysaccharides, rubbers or natural macromolecular compounds, are classified in subclass C08L.
Coating compositions and other polymer compositions for similar uses, e.g. paints, inks, woodstains and printing pastes, are classified in C09D.
C09D and C09J are seen as "related fields" of C08L - this structure has implications on search and classification.
For classification:
- If the claims only pertain to a "coating composition...", only the C09D symbols are given.
For searching: both C08G and C09D subclasses are to be searched, regardless of the wording of the claims, since in many documents of C08G, a passage relating to the use of the composition for coating can be found.
These rules apply in analogy for the adhesive compositions of C09J.
C09G covers the application of the compositions of C08L when used as polishes. Adhesives and adhesive processes are classified in C09J.
Derivatives of natural macromolecular polymers per se, e.g. derived from proteins or vulcanised oils, are classified in C08H.
Working-up, general processes of compounding and after-treatment are covered by subclass C08J. These include making solutions, dispersions etc., plasticising, compounding with additives, e.g. colouring or masterbatching, crosslinking, manufacture of articles or shaped materials, chemical treatment or coating of such articles, making porous, cellular or foamed materials, and recovery or working up of waste materials.
Materials used in applications not otherwise provided for, are classified in C09K. These include sealing or anti-slip materials, heat-transfer, heat-exchange or heat-storage materials, drilling compositions, luminescent or tenebrescent materials, etching, surface-brightening or pickling materials, antioxidant materials, soil-conditioning or soil-stabilising materials, liquid crystal or fireproofing materials.
The preparation for medical, dental or toilet purposes is classified in A61K.
Multiple Classification
Biocidal, pest repellant, pest attractant, or plant growth regulatory activity of chemical compounds or preparations is further classified in A01P.
Application of macromolecular compositions as biocides, pest-repellants, pest-attractants, or plant growth activity regulators is further classified in subclass A01N.
Therapeutic activity of macromolecular compounds is further classified in subclass A61P.
The use of cosmetics or similar toilet preparations is further classified in subclass A61Q.
Processes using enzymes or microorganisms in order to (i) liberate, separate or purify a pre-existing compound or composition, or to (ii) treat textiles or clean solid surfaces of materials, are further classified in subclass C12P.
Examples of places where the subject matter of this place is covered when specially adapted, used for a particular purpose, or incorporated in a larger system:
Application of macromolecular compositions as pesticides or herbicides | |
Application of macromolecular compositions as pharmaceutical compositions or cosmetics | |
Application for golf balls | |
Application for hollow fibres membranes | |
Application for tyres | |
Application of macromolecular compositions as explosive compositions | |
Application of macromolecular compositions in coating compositions | |
Application of macromolecular compositions in adhesive compositions | |
Application of macromolecular compositions in lubricants | |
Application for fibres | |
Application for non-woven fabrics | |
Application for the treatment of fibres with polymers | |
Application for tubes | |
Application for optical articles, optical parts, e.g. contact lenses | |
Application for optical elements e.g. polarizer | |
Application for cables or wires | |
Application for printed circuits, in particular photosensitive compositions |
Attention is drawn to the following places, which may be of interest for search:
Layered products | |
Liquid crystal compositions | |
Electrolytic processes, e.g. electrophoresis |
Classification guidance
- In this subclass, group C08G 18/00 takes precedence over all other groups. A further classification is given if the polymers are obtained by reactions forming specific linkages for which an appropriate group is provided.
- Within each main group of this subclass, in the absence of an indication to the contrary, classification is made in the last appropriate place.
- In groups C08G 61/00 - C08G 79/00, in the absence of an indication to the contrary, macromolecular compounds obtained by reactions forming two different linkages in the main chain are classified only according to the linkage present in excess as disclosed in the document.
- This subclass also covers compositions based on monomers which form macromolecular compounds classifiable in this subclass.
- If the monomers are defined, classification is made in groups C08G 2/00 - C08G 79/00, C08G 83/00 according to the polymer to be formed.
- If the monomers are defined in a way that a composition cannot be classified within one main group of this subclass, the monomers are classified in group C08G 85/00.
- If the compounding ingredients are of interest per se, classification is also made in subclass C08K.
Combination sets (C-Sets):
In this subclass, C-Sets classification is applied to the following groups, listed in the table below, if the document discloses a pertinent combination of technical features that cannot be covered by the allocation of a single symbol. The fourth column of the table indicates the place where the detailed information about the C-Sets construction and the associated syntax rules can be found, in the definition section "Special rules of classification".
C-Sets ID | Base Symbols | Subsequent Symbols | C-Sets Formula; Location of C-Sets Rules |
#C8Ga | C08G 18/2805, C08G 18/30 - C08G 18/3897, C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, C08G 18/70 - C08G 18/8096 | (C08G, C08G); reaction of a prepolymer with a reactive compound; see C08G 18/00. | |
#C8Gb | (C08G, C08G); oligomerisation of isocyanate- or isothiocyanate-terminated of prepolymers; see C08G 18/00. | ||
#C8Gc | C08G 18/67 - C08G 18/679, excluding C08G 18/6705 | (C08G, C08G); manufacture of polymers from unsaturated low-molecular-weight compounds having active hydrogens and the resulting polymer also containing ionic or ionogenic group; see C08G 18/00. | |
#C8Gd | C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, C08G 18/6705, C08G 18/6795 - C08G 18/698 | (C08G, C08G); reaction step of an unsaturated compound having active hydrogen(s) with an isocyanate-terminated prepolymer, the second symbol refers to the high-molecular weight reaction component of the prepolymer; see C08G 18/00. | |
#C8Ge | (C08G, C08G); manufacture of unsaturated isocyanate(s) or isothiocyanate(s) containing ionic or ionogenic groups; see C08G 18/00. | ||
#C8Gf | C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65-C08G 18/6696, C08G 18/6705, C08G 18/6795-C08G 18/698, C08G 18/65 - C08G 18/6696, C08G 18/6705, C08G 18/6795 - C08G 18/698 | (C08G, C08G); reaction step of a process involving an unsaturated isocyanate-terminated prepolymer with a high molecular weight compound having active hydrogen; see C08G 18/00. |
The specific C-Sets rule is located at only one place of the base symbol in the section "Special rules of classification" in the definition. If the C-Sets rule is applicable to all groups of a subclass, it is located at the subclass level only. If the same C-Sets rule is applicable to multiple groups or subgroups within the same subclass, the C-Sets rule is placed at the highest group or subgroup of the multiple groups.
In this place, the following terms or expressions are used with the meaning indicated:
Addition polymers | Polymers in which unsaturated monomer molecules join together to form a polymer in which the molecular formula of the repeat unit is identical (except for the double bond) with that of the monomer. |
Block polymers | Polymers formed by polymerization of monomers on to a macromolecule having groups capable of inducing the formation of new polymer chains bound at one or both ends of the starting macromolecule, or by polymerization using successively different catalyst types or successively different monomer systems without deactivating the intermediate polymer. |
Condensation polymers | Polymers in which water or some other simple molecule is eliminated from 2 or more monomer molecules as they combine to form the polymer or crosslinks between polymer chains. |
Copolymers | Usually denotes polymers of 2 chemically distinct monomers, and sometimes denotes terpolymers containing more than 2 types of monomer unit. |
Graft polymers | Macromolecular compounds obtained by polymerizing monomers on to preformed polymers or on to inorganic materials. Such preformed polymers could be rubbers, polysaccharides, condensation polymers, homopolymers or copolymers of the addition polymer type. |
In patent documents, the following abbreviations are often used:
CPET | Crystallised polyethylene terephthalate |
DABCO | 1,4-diazabicyclo-2,2,2-octane or triethylene diamine (amine catalyst for PU foams) |
DBP | Dibutyl phthalate |
DOP | Dioctyl phthalate |
HDI | Hexamethylene diisocyanate |
IPDI | Isophorone diisocyanate |
MDI | Diphenylmethane-4,4'-diisocyanate |
PBT | Polybutylene terephthalate |
PEEK | Polyetheretherketone |
PEG | Polyethylene glycol |
PEI | Polyetherimide |
PEK | Polyetherketone |
PEO | Polyethylene oxide |
PES | Polyethersulphone |
PET | Polyethylene terephthalate |
PPE | Polyphenylene ether |
PPS | Polyphenylene sulphide |
PPSU | Polyphenylene sulphone |
PUR | Polyurethane |
TETA | Triethylene tetramine |
TDI | Toluene diisocyanate |
This place covers:
- Addition polymers from aldehydes or ketones, i.e. polyacetals and copolyacetals
- Catalysts used for such polymerisation
- Post-polymerisation treatments of such resins.
- Polymerization of aldehydes or ketones initiated by wave energy or particle radiation
- Chemical modification of such resins by after-treatment
This place covers:
Polymerisation of acetaldehyde or cyclic oligomers thereof e.g. polymerisation of trioxane
This place covers:
- Condensation polymers of aldehydes or ketones with polyalcohols, e.g. the condensation product of formaldehyde and poly(alkylene oxides)
- Addition polymers of heterocyclic oxygen compounds containing in the ring at least once the grouping -O-C-O- , e.g. addition polymers of dioxolane, i.e. -
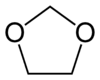 -
- .
.
This place does not cover:
Cyclic oligomers of aldehydes |
This place covers:
- condensation polymers of aldehydes only
- condensation polymers of ketones only
- condensation polymers of aldehydes with ketones only
This place covers:
Condensation polymers of aldehydes with ketones only, see for example WO2007141119 or US2005080222
This place covers:
In this place, the following terms or expressions are used with the meaning indicated:
Novolac resin | Phenol-formaldehyde resin where the molar ratio of formaldehyde to phenol of less than one, prepared in the presence of an acid catalyst |
Resol resin | Phenol-formaldehyde resin where the formaldehyde to phenol ratio is greater than one, prepared in the presence of a base |
This place covers:
For example
- Aminoplast resins, i.e. urea-formaldehyde (C08G 12/12)
- Melamine-formaldehyde (C08G 12/32) or
- Urea-melamine-formaldehyde (C08G 12/38)
This place does not cover:
Condensation polymers of aldehyde or ketone with aminophenol | |
Reaction of polyamides with aldehydes |
Within this main group, in the absence of an indication to the contrary, classification is made in the last appropriate place. This means that urea-melamine-formaldehyde resins are classified in C08G 12/38.
In patent documents, the following abbreviations are often used:
MF | Melamine-formaldehyde |
UF | Urea-formaldehyde |
This place covers:
for example
- Melamine-phenol-formaldehyde resins (C08G 14/10)
- Urea-phenol-formaldehyde resins (C08G 14/08), see EP2197928.
This place covers:
For example, condensation of aldehydes or ketones with natural products, oils, bitumens or residues.
This place does not cover:
Condensation polymers of aldehydes or ketones with polynitriles |
This place covers:
- Polyurethanes, polyureas and isocyanurates, i.e. polymeric products of isocyanates or isothiocyanates and compounds that are reactive towards isocyanates or isothiocyanates and some processes specific for these polymers.
Polymeric products containing ureide or urethane prepared without using isocyanate or isothiocyanate are classified in C08G 71/00.
Attention is drawn to the following places, which may be of interest for search:
Preparations for medical, dental or toilet purposes | |
Processes for applying liquid materials to surfaces | |
Shaping or joining plastics | |
Mould release agents | |
Layered products comprising polyurethanes | |
Preparation of isocyanates or isothiocyanates | |
Preparatory processes of porous or cellular materials, in which the monomers or catalysts are not specific | |
Working up of polyurethanes to porous or cellular articles | |
Use of inorganic or non-macromolecular organic substances as compounding ingredients | |
Coating compositions characterized by their physical nature or their effects produced | |
Adhesives processes | |
Materials for sealing |
Classification guidance
- In this group, for the purpose of groups C08G 18/28 - C08G 18/69, the addition of water for the preparation of cellular materials is not taken into consideration except in the case, wherein water is the only compound having active hydrogen C08G 18/302.
- When classification is done in C08G 18/00 for a specific monomer or a catalyst, the addition of water as the sole blowing agent is indicated by indexing code C08G 2110/0083. Moreover specific aggregation forms of water, e.g. absorbed water and water of crystallisation are also classified in C08J 9/02.
C-Sets classification:
- In C08G 18/00, C-Sets (e.g. # C8Ga, #C8Gb, #C8Gc, # C8Gd, # C8Ge, or #C8Gf) are used. The detailed information about the C-Sets construction and the associated syntax rules are set forth below.
- All exemplified compositions in a document should be classified as separate C-Sets. In the absence of examples, at least one C-Set is given on the basis of sufficient disclosure in the document.
Combination sets (C-Sets):
C-Sets statement: #C8Ga
- In group C08G 18/10, the reaction step of a process involving a prepolymer; which is obtained from a high molecular weight compound; with a compound having active hydrogen(s) is classified in the form of C-Sets.
- In group C08G 18/12, the reaction step of a process involving a prepolymer; which is obtained from two or more high molecular weight compounds; with a compound having active hydrogen(s) is classified in the form of C-Sets.
- Groups C08G 18/10 or C08G 18/12 are thus selected on the basis of the reaction leading to the prepolymer; whereas the C-Set reflects the reaction of said prepolymer with a compound having active hydrogen(s).In # C8Ga, the base symbol, representing the prepolymer; which is obtained from a single high molecular weight compound is taken from the group C08G 18/10, whereas the subsequent symbol representing the compound having active hydrogen(s) is taken from the groups C08G 18/2805, C08G 18/30 - C08G 18/3897, C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, or a polyisocyanate compound taken from the groups C08G 18/70 - C08G 18/8096.
- In #C8Ga , the base symbol, representing the prepolymer; which is obtained from two or more of high molecular weight compounds is taken from the group C08G 18/12, whereas the subsequent symbol representing the compound having active hydrogen(s) is taken from the groups C08G 18/2805, C08G 18/30 - C08G 18/3897, C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, or a polyisocyanate compound taken from the groups C08G 18/70 - C08G 18/8096.
- When the compounds having active hydrogens are taken in the range C08G 18/40 - C08G 18/64, the groups C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64 are thus used in the C-Sets as subsequent symbols and the appropriate corresponding subgroup thereof allocated as a separate single symbol.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant as it reflects the order of the process steps.
C-Sets examples:
C8Ga : A prepolymer (C08G 18/10) that is reacted with water (C08G 18/302) is classified as (C08G 18/10, C08G 18/302).
#C8Ga : A prepolymer (C08G 18/10) that is reacted with ethylene glycol (C08G 18/3206) is classified as (C08G 18/10, C08G 18/3206).
#C8Ga : A prepolymer (C08G 18/10) that is reacted with polyethylene glycol (C08G 18/4833) is classified as (C08G 18/10, C08G 18/48) and C08G 18/4833.
#C8Ga : A prepolymer which is obtained from two high molecular weight compounds (C08G 18/12) that is reacted with ethanol (C08G 18/282) is classified as (C08G 18/12, C08G 18/282).
#C8Ga : A prepolymer (C08G 18/10) that is reacted with toluene di-isocyanate (C08G 18/7621) is classified as (C08G 18/10, C08G 18/7621).
#C8Ga : A prepolymer obtained from the reaction of PEG and PPG with a diisocyanate molecule (C08G 18/12); that is reacted with a polycaprolactone (C08G 18/4277) is classified as (C08G 18/12, C08G 18/42) and C08G 18/4277.
C-Sets statement: #C8Gb
- In group C08G 18/10, the reaction step of a process involving the complete or partial oligomerisation of a prepolymer; which is obtained from a high molecular weight compound; is classified in the form of C-Sets.
- In group C08G 18/12, the reaction step of a process involving the complete or partial oligomerisation of a prepolymer; which is obtained from two or more high molecular weight compounds; is classified in the form of C-Sets.
- Groups C08G 18/10 or C08G 18/12 are thus selected on the basis of the reaction leading to the prepolymer; whereas the C-Set reflects the complete or partial oligomerisation of said prepolymer.
- In #C8Gb, the base symbol, representing the prepolymer; which is obtained from a single high molecular weight compound is taken from the group C08G 18/10, whereas the subsequent symbol representing the extent of reaction of the isocyanate or isothiocyanate is taken from the groups C08G 18/02 - C08G 18/027 and C08G 18/09 - C08G 18/097.
- In #C8Gb, the base symbol, representing the prepolymer; which is obtained from two or more of high molecular weight compounds is taken from the group C08G 18/12, whereas the subsequent symbol representing the reaction of the isocyanate or isothiocyanate is taken from the groups C08G 18/02 - C08G 18/027 and C08G 18/09 - C08G 18/097.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant as it reflects the order of the process steps.
C-Sets examples:
#C8Gb: An isocyanate-functionalized prepolymer (C08G 18/10) that is trimerized in the absence of a compound having active hydrogen into an isocyanurate compound (C08G 18/022) is classified as (C08G 18/10, C08G 18/022).
#C8Gb and #C8Ga : An isocyanate-functional prepolymer (C08G 18/10) that is trimerized in the presence of ethylene glycol into an isocyanurate compound (C08G 18/09) is classified as (C08G 18/10, C08G 18/09) (according to #C8Gb) and (C08G 18/10, C08G 18/3206) (according to # C8Ga).
C-Sets statement: #C8Gc
- In groups C08G 18/67 - C08G 18/679, excluding C08G 18/6705, the preparation of polymers containing ionic or ionogenic groups from unsaturated compounds is classified in the form of C-Sets.
- In #C8Gc, the base symbol, representing the unsaturated compound is taken from the groups C08G 18/67 - C08G 18/679, excluding C08G 18/6705, whereas the subsequent symbol representing the backbone is taken from the groups C08G 18/0804 - C08G 18/0833.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant.
C-Sets examples:
#C8Gc: The addition of hydroxyethyl acrylate (C08G 18/672) onto an isocyanate functional compound based on dimethylol propionic acid (C08G 18/348) is classified as (C08G 18/672, C08G 18/0823). Also allocate dimethylol propionic acid (C08G 18/348) as a single symbol.
#C8Gc and #C8Gd : The addition of hydroxyethyl acrylate (C08G 18/672) onto an isocyanate prepolymer based on a mixture of polyethylene glycol and dimethylol propionic acid (C08G 18/6692) is classified as (C08G 18/672, C08G 18/0823) according to #C8Gc and (C08G 18/672, C08G 18/6692) according to # C8Gd. Also allocate polyethylene glycol (C08G 18/4833) and dimethylol propionic acid (C08G 18/348) as single symbols.
C-Sets statement: #C8Gd
- In groups C08G 18/671 - C08G 18/672 the reaction of an unsaturated compound having active hydrogen(s) with a prepolymer; which is obtained from a high molecular weight compound; is classified in the form of C-Sets.
- In #C8Gd , the base symbol, representing the unsaturated compound is taken from the groups C08G 18/671 - C08G 18/672, whereas the subsequent symbol representing the backbone of the high molecular weight compound is taken from the groups C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, C08G 18/6705 and C08G 18/6795 - C08G 18/698.
- When the high molecular weight compounds used to make the isocyanate-functional or isothiocyanate-functional prepolymer are taken in the range C08G 18/40 - C08G 18/64, the groups C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64 are thus used in the C-Sets as subsequent symbols and as well as with the appropriate corresponding subgroup as a separate single symbol.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant.
C-Sets examples:
#C8Gd : The addition of hydroxyethyl methacrylate (C08G 18/672) onto an isocyanate-terminated polyethylene glycol (C08G 18/4833) is classified as (C08G 18/672, C08G 18/48) and C08G 18/4833.
C-Sets statement: #C8Ge
- In groups C08G 18/81 - C08G 18/8191, the preparation of unsaturated polymers containing ionic or ionogenic groups is classified in the form of C-Sets.
- In #C8Ge , the base symbol is taken from the groups C08G 18/81 - C08G 18/8191, whereas the subsequent symbol is taken from the groups C08G 18/0804 - C08G 18/0833.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant.
C-Sets examples:
#C8Ge : The addition of isocyanatoethyl methacrylate (C08G 18/8116) to dimethylol propionic acid is classified as (C08G 18/8116, C08G 18/0823) according to # C8Ge. Also allocate dimethylol propionic acid (C08G 18/348) as a single symbol.
#C8Ge and #C8Gf: The addition of isocyanatoethyl methacrylate (C08G 18/8116) to a mixture of polyethylene glycol and dimethylol propionic acid is classified as (C08G 18/8116, C08G 18/0823) according to # C8Ge and (C08G 18/8116, C08G 18/6692) according to #C8Gf. Also allocate polyethylene glycol (C08G 18/4833) and dimethylol propionic acid (C08G 18/348) as single symbols.
C-Sets statement: #C8Gf
- In groups C08G 18/8158 - C08G 18/8175, a process involving the reaction step of an unsaturated isocyanate-terminated compound with a high molecular weight compound having active hydrogens is classified in the form of C-Sets.
- In #C8Gf, the base symbol, representing the unsaturated isocyanate compound is taken from the groups C08G 18/8158 - C08G 18/8175, whereas the subsequent symbol representing the backbone of the polymer is taken from the groups C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64, C08G 18/65 - C08G 18/6696, C08G 18/6705 and C08G 18/6795 - C08G 18/698.
- When the prepolymer compounds are taken in the range C08G 18/40 - C08G 18/64, the groups C08G 18/40, C08G 18/42, C08G 18/44, C08G 18/46, C08G 18/48, C08G 18/50, C08G 18/52, C08G 18/54, C08G 18/56, C08G 18/58, C08G 18/60, C08G 18/61, C08G 18/62, C08G 18/63, C08G 18/64 are thus used in the C-Sets as subsequent symbols and as well as with the appropriate corresponding subgroup as a separate single symbol.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols.
- Duplicate symbols are not allowed in these C-Sets.
- The order of symbols in these C-Sets is relevant.
C-Sets examples:
#C8Gf: The addition of isocyanatoethyl methacrylate (C08G 18/8116) onto polyethylene glycol (C08G 18/4833) is classified as (C08G 18/8116, C08G 18/48) and C08G 18/4833.
C-Sets searches:
C-Sets search queries may be made according to C-Sets classification rules described in C08G 18/00.
In patent documents, the following abbreviations are often used:
CPP | Copolymer polyol |
DABCO | 1,4-Diazabicyclo(2.2.2)octane |
DMPA | Dimethylol propionic acid |
EDA | Ethylene diamine |
EO | Ethylene oxide |
HDI | Hexane diisocyanate |
H12MDI | Dicyclohexylmethane diisocyanate |
IEM | Isocyanato ethyl methacrylate |
IPDI | Isophorone diisocyanate |
JEFFAMINE | Amine capped polyether |
MDI | 4,4-Methylenebis(phenyl)isocyanate |
PEG | Polyethyleneglycol |
PIR | Polyisocyanurate |
PMDI | Polymethylene poly(phenylisocyanate) |
PO | Propylene oxide |
PPG | Polypropylene glycol |
PTMO | Polytetramethylene oxide |
TDI | Toluene diisocyanate |
TMP | Trimethylol propane |
TMXDI | Tetramethylxylylene diisocyanate |
TPU | Thermoplastic polyurethane |
XDI | Xylylene diisocyanate |
This place covers:
- Polymerisation of isocyanates or isothiocyanates in the absence of compounds that are reactive towards isocyanate or isothiocyanate.
This place does not cover:
Oligomerisation in the presence of compounds that are reactive towards isocyanate | |
Use of oligomerised isocyanates | |
Oligomerised isocyanates per se |
This place covers:
Process features such as catalysts which are specific for polymeric products of isocyanates or isothiocyanates and are not covered elsewhere
This place does not cover:
Working-up of polymeric products of isocyanates or isothiocyanates to foams |
This place covers:
Oligomerisation of isocyanates in the presence of compounds that are reactive towards isocyanate
This place does not cover:
Polymerisation of isocyanates in the absence of compounds that are reactive towards isocyanate | |
Use of oligomerized isocyanates |
Additional information: oligomerisation to isocyanurate groups are classified in C08G 2115/02.
This place covers:
Prepolymer processes involving reaction of isocyanates or isothiocyanates with compounds having active hydrogen having a high molecular weight (more than 10 repeating monomer units or a molecular weight higher than 500) in a first reaction step.
Attention is drawn to the following places, which may be of interest for search:
Isocyanates or isothiocyanates reacted with low molecular weight active hydrogen compounds ; Masked polyisocyanates |
C-Sets classification
In C08G 18/10 and C08G 18/12, C-Sets (e.g. # C8Ga, #C8Gb) are used. The detailed information about the C-Sets construction and the associated syntax rules are found in the "Special rules of classification" in C08G 18/00.
C-Sets searches:
C-Sets search queries may be made according to C-Sets classification rules described in C08G 18/00.
This place covers:
Compounds containing active hydrogen and having a molecular weight of less than 500 or have less than 10 repeating monomer units.
C08G 18/0838 takes precedence over this subgroup.
This place covers:
Compounds having more than one group containing active hydrogen.
This place covers:
Compounds having more than one group containing active hydrogen and having a molecular weight of more than 500 or having more than 10 repeating monomer units.
C08G 18/0838 takes precedence over this subgroup.
This place covers:
What is known in industry as, "polymer polyols": polymers containing active hydrogen (polyol) with particles prepared from polymerised carbon to carbon unsaturated monomers grafted onto the active hydrogen polymer or forming a block copolymer with the active hydrogen polymer.
The polymerised carbon to carbon unsaturated monomer particles are dispersed in the active hydrogen containing polymer (polyol) which is reactive towards isocyanate. The polymerised carbon to carbon unsaturated monomer particles themselves are therefore not reactive towards isocyanate.
This place does not cover:
Macromolecular compounds obtained by polymerising monomers on to polymers of C08G |
This place covers:
Mixtures of low molecular weight compounds having active hydrogen with high molecular weight compounds (more than 10 repeating monomer units or a molecular weight higher than 500) having active hydrogen where either the low molecular weight (less than 10 repeating monomer units or a molecular weight lower than 500) compound is essential for the invention or where the high molecular weight (more than 10 repeating monomer units or a molecular weight heigher than 500) compound is not a compound of groups C08G 18/42 , C08G 18/48 or C08G 18/52
C08G 18/0838 takes precedence over this subgroup.
This place covers:
Mixtures of low molecular weight compounds having active hydrogen with high molecular weight compounds having active hydrogen from the groups C08G 18/42 , C08G 18/48 or C08G 18/52
This place covers:
Compounds that have Carbon to Carbon unsaturation and groups that are reactive towards isocyanate/isothiocyanate.
C-Sets classification:
In C08G 18/67 - C08G 18/679 and C08G 18/671 - C08G 18/672, C-Sets (e.g. #C8Gc, # C8Gd) are used. The detailed information about the C-Sets construction and the associated syntax rules are found in the "Special rules of classification" in C08G 18/00.
C-Sets searches:
C-Sets search queries may be made according to C-Sets classification rules described in C08G 18/00.
This place covers:
Use of oligomerised isocyanates.
This place does not cover:
Oligomerisation of isocyanates in the absence of compounds that are reactive towards isocyanate | |
Oligomerisation in the presence of compounds that are reactive towards isocyanate | |
Oligomerised isocyanates per se |
This place covers:
Blocked polyisocyanates or polyisocyanates prereacted with low molecular weight compounds having active hydrogen.
This place does not cover:
Prepolymers, i.e. polyisocyanates prereacted with high molecular weight compounds having active hydrogen |
This place covers:
Unsaturated iso(thio)cyanates and poly(thio)isocyanates masked with unsaturated compounds having active hydrogen
C-Sets classification:
In C08G 18/81 - C08G 18/8191 and C08G 18/8158 - C08G 18/8175, C-Sets (e.g. #C8Gf, # C8Ge) are used. The detailed information about the C-Sets construction and the associated syntax rules are found in the "Special rules of classification" in C08G 18/00.
C-Sets searches:
C-Sets search queries may be made according to C-Sets classification rules described in C08G 18/00.
This place covers:
Chemical modification of polyurethanes other than through reaction with isocyanate or isothiocyanate
This place does not cover:
Compositions of unspecified macromolecular compounds having specific groups |
This place covers:
Epoxy resins, i.e. all polycondensates having more than one epoxy groups per molecule (diepoxides and polyepoxides).
Epoxy resins characterized by special parameters.
The use or choice of inorganic or non-macromolecular organic materials as compounding agents are classified in subclass C08K.
Compositions of macromolecular compounds, either with other macromolecular compounds or with other ingredients, including compositions of polysaccharides, rubbers or natural macromolecular compounds, are classified in subclass C08L.
Coating compositions and other polymer compositions for similar uses, e.g. paints, inks, woodstains and printing pastes, are classified in subclass C09D.
Adhesives and adhesive processes are classified in subclass C09J.
Materials for applications not otherwise provided for, or applications of materials not otherwise provided for, are classified in subclass C09K. These include sealing or anti-slip materials, heat-transfer, heat-exchange or heat-storage materials, drilling compositions, luminescent or tenebrescent materials, etching, surface-brightening or pickling materials, antioxidant materials, soil-conditioning or soil-stabilising materials, liquid crystal or fireproofing materials.
This place does not cover:
Low-molecular-weight polyepoxy compounds | |
Monoepoxide compounds, e.g. oxiranes or preparation of oxiranes |
Attention is drawn to the following places, which may be of interest for search:
Polymers containing ether groups, e.g. oxetanes | |
Polymers containing S- link, e.g. thiiranes | |
Compositions of homo- or copolymers of acrylic or methacrylic esters having pendent glycidyl groups |
No Indexing Codes are used in this group.
Last place rule:
When an epoxy composition comprises a special hardener, or mixtures of special hardeners, catalysts or characteristic epoxy resins, classification is given in C08L 63/00, but also the corresponding classes in subgroups of C08G 59/00, since C08G 59/00 is much more detailed.
In this place, the following terms or expressions are used with the meaning indicated:
Epoxy resins | All polycondensates having more than one epoxy groups per molecule |
Bisphenol A | 4,4'-(Propane-2,2-diyl)diphenol |
Bisphenol F | 2-[(2-Hydroxyphenyl)methyl]phenol |
Bisphenol S | 4-(4-Hydroxyphenyl)sulfonylphenol |
DGEBA | Diglycidyl ether of bisphenol A |
Epoxide | Oxirane |
Glycidyl- | 2,3-Epoxypropyl- |
Hardener | Crosslinker |
This place covers:
The preparation of epoxy resins, in a general way.
This place does not cover:
Chemical after-treatment of diepoxides or polyepoxides | |
Polymers obtained by pre-reaction of diepoxides or polyepoxides with curing agents |
This place covers:
Polycondensates containing more than one epoxy group per molecule, characterised by the preparation or apparatus used.
This place does not cover:
Chemical after-treatment of diepoxides or polyepoxides | |
Polymers obtained by pre-reaction of diepoxides or polyepoxides with curing agents |
This place covers:
Polycondensates containing more than one epoxy group per molecule, characterized by the purification methods.
This place does not cover:
Chemical after-treatment of diepoxides or polyepoxides | |
Polymers obtained by pre-reaction of diepoxides or polyepoxides with curing agents |
This place covers:
Preparation of polycondensates containing more than one epoxy group per molecule, where an unsaturated precursor is epoxydized, e.g. by an oxidative step.
This place does not cover:
Chemical after-treatment of diepoxides or polyepoxides | |
Polymers obtained by pre-reaction of diepoxides or polyepoxides with curing agents |
This place covers:
The modification of epoxy resins, by further reaction with organic or inorganic compounds.
This place does not cover:
Epoxy resins obtained by unsaturated monomeric or polymeric precusors | |
Polymers obtained by pre-reaction of diepoxides or polyepoxides with curing agents |
This place covers:
Polymers obtained by polycondensation of epoxy resins with curing agents or catalysts
Advancement polymers having end epoxy groups.
This place does not cover:
Macromolecules obtained by epoxydation of unsaturated precursor, e.g. polymer or monomer | |
Chemical after-treatment of diepoxides or polyepoxides |
This place covers:
- Polymers obtained by reactions forming a carbon-to-carbon link in the main chain otherwise than by addition polymerisation reactions only involving carbon-to-carbon unsaturated bonds (wherein in the latter case the reactive carbon-carbon group stays intact without cleavage of fragments). The polymers included in this main group are typically prepared by means of polycondensation reactions.
- Polyphenylenes
- Polyxylylenes
- Polyfluorenes
- Polynorbornenes prepared by ring-opening metathesis reactions
- Poly(ether ketone ketones) prepared from diacid chloride compounds and aryl comonomers by means of Friedel-Crafts reactions (classified in C08G 61/127)
- Polymers prepared by polycondensation reactions involving the reactions between aryl compounds and co-monomers containing methylol groups or protected methylol groups or chloromethyl moieties.
Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds under polyaddition reactions wherein the reactive carbon-carbon group stays intact without cleavage of fragments are classified in subclass C08F.
Corresponding main groups for the polymers according to main group C08G 61/00 can be found in the main groups C08L 65/00 (compositions based on such polymers), C09D 165/00 (coating compositions based on said polymers), and C09J 165/00 (adhesive compositions based on such polymers).
Relationship with main groups of the same subclass C08G:
Polymers prepared by condensation reactions of aldehydes or ketones with phenols only are classified in groups C08G 8/00 - C08G 8/38, since C08G 2/00 - C08G 16/00 takes precedence. For the same reasons, condensation polymers of aldehydes or ketones only are classified in C08G 6/00 - C08G 6/02. Polymers which may otherwise be formed by carbon-carbon bond formation, but which are prepared by condensation reactions other than those involving the formation of carbon-carbon bonds in the main chain, are classified in the appropriate groups, e.g. C08G 73/0611 for polypyrroles formed from amines and polyketones. Polyketones are classified in C08G 67/02.
This place does not cover:
Production of polymers using enzymes |
Attention is drawn to the following places, which may be of interest for search:
Condensation polymers of aldehydes with phenols only | |
Condensation polymers of aldehydes with aromatic hydrocarbons or halogenated aromatic hydrocarbons only | |
Poly(ether ketones) obtained by reactions forming an ether link in the main chain of the macromolecule | |
Polycondensates having nitrogen-containing heterocyclic rings in the main chain of the macromolecules obtained by reactions forming a linkage containing nitrogen, including polypyrroles | |
Complementary pieces of information concerning C08G 61/00 | |
Catalysts in general | |
Polyacetylenes prepared by polyaddition | |
Compositions, coating compositions and adhesive compositions based on polymers according to main group C08G 61/00 are classified in main groups | |
Conductors characterised by the conductive material: Intrinsically conductive polymers | |
Solid state devices using oligomeric or polymeric organic materials as the active part, or using a combination of organic materials including organic oligomers or polymers with other materials as the active part |
In the subclass C08G, main group C08G 18/00 takes precedence over all other groups. A further classification is given if the polymers are obtained by reactions forming specific linkages for which an appropriate group is provided.
Within each main group of this subclass, in the absence of an indication to the contrary, classification is made in the last appropriate place.
In main groups C08G 61/00-C08G 79/00, in the absence of an indication to the contrary, macromolecular compounds obtained by reactions forming two different linkages in the main chain are classified only according to the linkage present in excess.
In the subgroup C08G 61/12, the following peculiarities apply:
For polymers comprising different heterocyclic constituents in the polymer main chain, a classification will be put for each. For example, a polymer consisting of thiophene, pyrrole, and triphenylamine in polymerised form will be classified in C08G 61/12 for the triphenylamine, C08G 61/124 and C08G 61/126.
Polymers according to C08G 61/00 which have been obtained from five-membered heterocyclic monomers comprising more than one heteroatom in the heterocycle will be classified in C08G 61/123.
When the macromolecular compounds are formed from condensed heterocyclic monomers, e.g. 2,1,3-benzothiadiazole, which comprise a five- or six-membered heterocycle, such a compound would still be considered derived from five- or six-membered heterocyclic compounds.
For example, a polymer derived from a 2,1,3-benzothiadiazole starting compound would be classified in C08G 61/123. Complementary structural aspects, such as codification of condensed heterocycles, are classified in C08G 2261/30 -C08G 2261/376.
When assigning the main group C08G 61/00 or subgroups thereof to a document, classification in the main group C08G 2261/00 and/or subgroups thereof is obligatory.
In the absence of an indication to the contrary, classification is made in the last appropriate place within C08G 2261/00 and subgroups. Classification in this main group is obligatory when classes in C08G 61/00 and subgroups thereof are assigned to a document.
3,4-Ethylenedioxythiophene in polymerised form is classified in C08G 2261/1424 plus C08G 2261/3223 and not in C08G 2261/344 (the aspect of cyclised ether side-chain is prominent).
C08G 2261/46 is only used as an additional symbol for classifying Diels-Alder crosslinking reactions of polymers prepared by reactions falling within the scope of C08G 61/00 - C08G 61/127 (since polymerisations effected by Diels-Alder cycloadditions are polyaddition reactions per se covered by subclass C08F of the classification scheme).
In C08G 2261/30, the following peculiarities apply: For polymers comprising different heterocyclic constituents in the polymer main chain, a classification will be entered for each. E.g. a polymer consisting of thiophene, pyrrole, and triphenylamine in polymerised form will be classified in C08G 2261/3162 (triphenylamine), C08G 2261/3221 (pyrrole), and C08G 2261/3223 (thiophene).
When the macromolecular compounds are formed from condensed heteroaromatic monomers which comprise various aromatic heterocycles, each heterocycle will be classified (of course as condensed ring system). For example, thieno[3,4-b]pyrazine in polymerised form will be classified in C08G 2261/3243 (a condensed thiophene unit) and in C08G 2261/3241 (standing for the condensed pyrazine ring).
In condensed aromatic ring systems comprising aromatic and heteroaromatic condensed rings only the heteroaromatic rings will be specified in C08G 2261/00 - C08G 2261/964. For example, benzo[c]thiophene in polymerised form will be classified in C08G 2261/3243.
When partially aromatic (or heteroaromatic) structural elements are incorporated into the polymeric main chain, which can be broken down into smaller main chain constituents, the latter should also be classified (unless specific pertinent subgroups such as C08G 2261/3424 or C08G 2261/3442 exist):
E.g. a 2,5-diethinylthiophene monomeric unit should be classified in C08G 2261/344, C08G 2261/3223, and in C08G 2261/3328 (since the polymer could have been prepared from thienyl and ethinyl monomers instead).
In this place, the following terms or expressions are used with the meaning indicated:
Addition polymer | Polymer which is formed by an addition reaction, where monomers bond together via rearrangement of bonds without the loss of any atom or molecule. This is in contrast to a condensation polymer which is formed by a condensation reaction where a molecule, such as water, is cleaved off during the formation. |
Condensation polymer | Polymer in which water or some other simple molecule is eliminated from 2 or more monomer molecules as they combine to form the polymer. |
In patent documents, the following abbreviations are often used:
ADMET | Acyclic diene metathesis |
ROMP | Ring-opening metathesis polymerisation |
This place covers:
Polymeric products containing ester bonds in the backbone.
This place does not cover:
Polyester-urethanes | |
Polycarbonates | |
Polyester-amides | |
Polyester-imides | |
Block- and graft copolymers containing polyester and polysiloxane sequences | |
Block or graft copolymers containing ester bonds and sequences of polymers of C08C and C08F | |
Polymers based on ethylenically unsaturated monomers containing ester bonds in the side chain | C08F 20/00, C08F 120/00, C08F 220/00, C08F 18/00, C08F 118/00, C08F 218/00 |
Graft polymers obtained by polymerizing unsaturated monomers on polyesters | |
Polyhydroxycarboxylic acids obtained by fermentation or enzyme-using processes |
Attention is drawn to the following places, which may be of interest for search:
Dendrimers, hyperbranched polymers, polyrotaxanes, polycatenanes or supramolecular polymers | |
Preparation of medical dental or toilet purposes | |
Chemical aspects of and materials for bandages, dressings, absorbent pads or surgical articles | |
Layered products comprising polyesters | |
Polyhydroxy compounds | |
Polycarboxylic or hydrocarboxylic acids | |
Use of inorganic or non-macromolecular organic substances a compounding ingredients | (C08K 3/00, C08L 67/00)-( C08K 3/00, C08L 67/08), ( C08K 5/00, C08L 67/00)-( C08K 5/00, C08L 67/08) |
Degradable polymer compositions | |
Coating compositions characterized by their physical nature or their effects produced | |
Polyester fibres | |
Binders for toners |
This place covers:
Polyester not provided for in groups C08G 63/06 - C08G 63/6988.
This place covers:
Polyesters containing sequences obtained by polycondensation of one or more ω-hydroxycarboxylic acid derivatives, e.g. hydroxyalkanoates
Attention is drawn to the following places, which may be of interest for search:
Polyhydroxycarboxylic acids containing oxygen in the form of ether groups | |
Polyhydroxycarboxylic acids containing atoms other than carbon, hydrogen and oxygen |
This place covers:
Polyesters containing sequences obtained by ring-opening of one or more cyclic esters, e.g. polylactic acid or ε-caprolactone.
Attention is drawn to the following places, which may be of interest for search:
Processes for the preparation of polylactones and polylactides characterized by the catalyst used |
This place covers:
Polyesters derived from polycarboxylic acids and polyhydroxy compounds which have been prepared in the presence of 10 wt% or more of ester forming compounds having more than two reactive groups.
This place does not cover:
Polyesters having been prepared in the presence of less than 10 wt% of compounds having more than two reactive groups | |
Polyesters containing oxygen in the form of ether groups | |
Polyesters containing atoms other than carbon, hydrogen and oxygen | |
Polyesters modified by chemical after-treatment |
This place covers:
Polyesters containing sequences obtained by polycondensation of one or more dicarboxylic acids and one or more dihydroxy compounds.
This place does not cover:
Polyesters containing oxygen in the form of ether groups | |
Polyesters containing atoms other than carbon, hydrogen and oxygen | |
Polyesters modified by chemical after-treatment |
This place covers:
Polyesters derived from dicarboxylic acids and dihydroxy compounds which have been prepared in the presence of less than 10 wt% of compounds having one reactive group or more than two reactive groups.
This place does not cover:
Polyesters derived from dicarboxylic acids and dihydroxy compounds which have been prepared in the presence of 10 wt% or more of compounds having more than two reactive groups |
This place covers:
Polyesters chemically modified by esterification with unsaturated monoacids or monoalcohols.
This place does not cover:
Polymeric reaction products of polyesters which are chemically modified by esterification with unsaturated acids or alcohols with ethylenically unsaturated compounds |
Attention is drawn to the following places, which may be of interest for search:
Polyesters derived from polycarboxylic acids and polyhydroxy compounds modified by chemical after-treatment |
This place covers:
Polymers containing carboxylic ester groups and carbonate groups, even if the carbonate groups are in excess.
This place covers:
Polyesters containing ether groups of any kind, e.g. sugar moieties, polyalkylene oxide sequences.
Attention is drawn to the following places, which may be of interest for search:
Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule |
C08G 63/42 and C08G 63/58 take precedence over C08G 63/66.
This place covers:
Polyesters containing heteroatoms at any place in the side- or main chain
This place covers:
Preparation processes in which the process or a step thereof is characterized by the apparatus or a feature thereof
Attention is drawn to the following places, which may be of interest for search:
Apparatuses for preparing polymers | |
Extrusion molding |
This place covers:
Processes in which the preparation of polylactones or polylactides is characterized by the catalyst used.
This place does not cover:
Polylactones or polylactides |
This place covers:
Polymeric products containing carbonate bonds in the backbone.
This place does not cover:
Polycarbonate-urethanes | |
Polyesters | |
Polycarbonates containing ester groups in the backbone | |
Polycarbonate-amides | |
Polycarbonate-imides |
Attention is drawn to the following places, which may be of interest for search:
Dendrimers, hyperbranched polymers, polyrotaxanes, polycatenanes or supramolecular polymers | |
Layered products comprising polycarbonates | |
Carbonates | |
Use of inorganic or non-macromolecular organic substances a compounding ingredients | (C08K 3/00, C08L 69/00(B))( C08K 5/00, C08L 69/00(B)) |
Polycarbonate fibres | |
Polycarbonate lenses | |
Polycarbonate binders for toners | |
Polycarbonate record carriers |
This place covers:
Block- and graft copolymers containing polycarbonate sequences and sequences of polymers of C08G.
This place does not cover:
Attention is drawn to the following places, which may be of interest for search:
Block- or graft polymers containing polycarbonate and polysiloxane sequences |
This place covers:
Preparation of polyether by ring opening reaction of cyclic ether in the presence of "other compound" e.g. active H containing compound which acts as an initiator for polymerisation. e.g. R-OH + n ethylene oxide → R-(O-CH2-CH2)n
Attention is drawn to the following places, which may be of interest for search:
Preparation of ethers by reaction of an oxirane with hydroxy group |
(1) If the "other compound" contains two different active H containing groups, the compound should be classified in both relevant groups e.g. aminoethanol should be classified in C08G 65/2609 and C08G 65/2624.
(2) If the "other compound" is sugar or polysaccharide containing OH groups, classification should be made in C08G 65/2606.
(3) Aniline is classified in C08G 65/2627 which reads " Aromatic or arylaliphatic amine group" .
(4) Pyridine or piperazine are classified in C08G 65/263 which reads "Heterocyclic amine" .
This place covers:
Compounds characterised by the catalyst used in the ring opening reaction between a cyclic ether and an "other compound".
Attention is drawn to the following places, which may be of interest for search:
Catalysts per se | |
Cyanide catalysts |
(1) Classification is made according to the metal in the catalyst if any.
(2) Boron is considered a metal.
(3) Magnesium is to be considered an alkaline earth metal.
(4) If a catalyst is classified in C08G 65/269 (mixed catalyst systems), then separate components should be classified as well; for example,
(5) If a catalyst should be classified in C08G 65/2693 – then if possible both components should be classified, e.g. aluminium supported on clay based catalyst is classified in C08G 65/2693, C08G 65/2654 and C08G 65/2657.
In this place, the following terms or expressions are used with the meaning indicated:
DMC | Double Metal Cyanide |
This place covers:
Post-polymerisation treatment of cyclic ethers made exclusively by ring opening reactions of cyclic ethers, e.g. recovery, purification, drying or removal of catalyst residues even if done by chemical means for example acidification.
Attention is drawn to the following places, which may be of interest for search:
Separation or purification of ethers |
This place covers:
Polyethers made from phenols and other compounds e.g. OH-Ar-OH + X-Ar-X where X is a halogen leaving groups. It encompasses aromatic (Ar) polyethers or polyetherketones.
This place does not cover:
Polyetherketones made by Friedel -Krafts acylation | |
Polyphenylene ether/oxide | |
Polyketones from carbon monoxide | |
Polyetherimides | |
Polythioether-ethers | C08G75/15 |
Polyethersulphones |
Attention is drawn to the following places, which may be of interest for search:
Polyethersuphones |
In this place, the following terms or expressions are used with the meaning indicated:
PEK | Polyetherketones |
PEEK | Polyetheretherketones |
PES | Polyethersulphones |
PEI | Polyetherimides |
PAEK | Polyaryletherketones |
PAES | Polyarylethersulphones |
This place covers:
- Copolymers of carbon monoxide and aliphatic unsaturated compounds
- Polyanhydrides
This place covers:
Polyketones made by reaction of from carbon monoxide with aliphatic unsaturated compounds
This place does not cover:
Polyetherketones made by Friedel-Krafts acylation | |
Polyaryletherketones |
This place covers:
Polymers containing the following repeat unit:
 .
.
This place covers:
Polyamides derived from
amino-carboxylic acids, e.g. alpha-amino-carboxylic acids
lactams, e.g. beta-lactams
from polyamines and polycarboxylic acids
Pyrrolidones or piperidones
Polyester-amides
Preparations of above polymers
Post-polymerisation treatment or polymers modified by chemical after-treatment
This place does not cover:
Products obtained from isocyanates or isothiocyanates | |
Polysuccinimides | |
Polyamide-imides | |
Artificial filaments or fibres | |
Treatment of textiles |
In patent documents, the following words/expressions are often used as synonyms:
- "Polycaprolactam" and "Nylon 6"
- "Aramid" and "aromatic polyamide"
This place covers:
Polyamines, e.g. polyethyleneimines
Polyhydrazides, polytriazoles, polyamino-triazoles or polyoxadiazoles
Polyimides, polyester-imides or polyamide-imides
Unsaturated polyimide precursors
Polybenzimidazoles
Polybenzoxazoles
This place does not cover:
Polycarbodiimides prepared from isocyanates | |
Macromolecular compounds obtained by reactions forming a carbon-to-carbon link in the main chain of the macromolecule, derived from five-membered heterocyclic compounds, containing one nitrogen atom in the ring | |
Polythiazoles |
The IPC group C08G73/04 is not used, group C08G 73/0206 or subgroups are used instead.
This place covers:
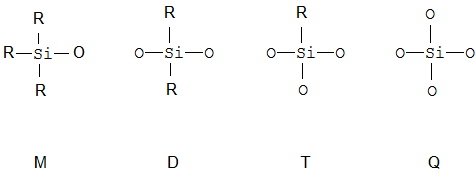
Polymers where there is a Si atom in the main chain; they are referred to with the MDTQ nomenclature.
Compositions of polymers containing Si in the main chain and other polymers are classified in C08L 83/00.
Coating of polymers containing Si in the main chain are classified in C09D 183/00 and adhesives of polymers containing Si in the main chain are classified in C09J 183/00.
Attention is drawn to the following places, which may be of interest for search:
Application for medical or pharmaceutical purposes | |
Application in cosmetics | |
Application in layered products | |
Application to construction materials | |
Preparation of aqueous siloxane emulsions | |
Manufacturing of foams | |
Compounding ingredients | |
Compositions of polymers of other C08L groups | |
Application of siloxanes as pressure sensitive adhesives, i.e. PSA | |
Release coating composition on which the PSA is applied | |
Treating fibres and yarns | |
Application in optical articles, optical parts, e.g. contact lenses | |
Application in semiconductors e.g. as dielectric layer or encapsulation |
When classifying within this group, a distinction has to be made structurally between polysilicates and siloxanes which contain Si-R groups, such as polymers, which contain only D-units, or resins which contain at least one branching unit such as T or Q.
Polysilicates are in C08G 77/02, all kind of other polymers or resins are in C08G 77/04 or its sub-groups.
It is obligatory to add the following additional indexing codes (CAA) where applicable:
- C08G 77/70 for every document which uses the MDTQ nomenclature in the claims or the examples
- C08G 77/80 for polysiloxanes having aromatic substituents such as phenyl side groups.
In this place, the following terms or expressions are used with the meaning indicated:
Condensation cure | The cure is established via condensation reactions such as Si-OR + HO-Si → Si-O-Si or Si-OH + HO-Si → Si-O-Si, e.g. HOMDxMOH + (RO)3SiR → elastomer which is performed with the help of a variety of condensation catalysts, e.g. tin compounds, acids or bases. |
Curing systems | The three most important ways to harden or cure siloxanes are hydrosilation-, condensation- or radical cure |
Hydrosilation cure | The cure is established via the hydrosilation (or hydrosilylation or addition) reactionSi-CH=CH2 + H-Si → Si-CH2-CH2-Si , e.g. ViMDxMVi + MDH3DxM → elastomeric material (3d x-linked), which is done in most cases with the help of a platinum catalyst, e.g. platinic acid, platinum compounds or Karstedt catalyst. |
MDTQ nomenclature | The so called MDTQ nomenclature exists to facilitate the description of siloxane molecules. |
MDTQ-resin | Contain all four elements |
MQ-resin | A resin which contains M and Q units, i.e. prepared from tetraalkoxysilanes, e.g. TEOS and monoalkoxysilanes |
Radical or peroxide cure | The cure is established via the reaction Si-CH3 + CH3-Si → Si-CH2-CH2-Si which is done in most cases with the help of a peroxide catalyst. |
Silsesquioxane | Resin which falls under the stochiometric formula RSiO3/2 (silsesqui means one and a half), e.g. a T-resin |
T-resin | A branched structure which contains only T-units, i.e. is prepared from trialkoxysilanes or trichlorosilanes |
Polysiloxanes with organic subsitiuents on the Si-O backbone are also commonly referred to in the literature as "polyorganosiloxanes" and "organopolysiloxanes". The terms "siloxanes" and "silocones" are also commonly encountered for these compounds.
MDxM | Non functional PDMS, i.e. polydimethylsiloxane |
MM | Hexamethyldisiloxane |
ViMDxMVi | PDMS having vinyl end groups |
MDHxDxM | PDMS having SiH side groups |
In patent documents the following expressions
"platin+" or" karstedt" are often used as synonyms when searching for "platinum catalyst";
((alkoxy 2d cur+), (condens+ 2d cur+), tin+, stannous+ or (moisture 2d cur+)) are often used as synonyms when searching for condensation catalysts;
perox+ is often used when searching for radical or peroxide catalysts
This place covers:
Polymers containing Si in the main chain where only Q groups are present, with no organic groups attached to the siloxane backbone, e.g. synthesis of polymers or gels via tetraethoxy orthosilicate (TEOS) condensation reactions.
This place does not cover:
Synthesis of silica particles |
This place covers:
Polysiloxanes, i.e. at least one M, D or T group present, e.g. T-resins, MQ-resins, D-polymers or silsesquioxanes, with more than 25 silicon atoms.
This group is used when a no more relevant group can be found.
This place covers:
Polysiloxanes with at least five silicon atoms present, e.g. cyclosiloxanes, polyhedral silsesquioxane (POSS, T8 cubes), or oligomers.
This place does not cover:
Polysiloxanes containing fewer than five units |
This place covers:
Processes referred to as redistribution, polymerization-deploymerizatiohn, resizing.
This place covers:
Polysiloxanes where the O atom is present in the substituents and not the backbone, e.g. direct or no direct silicon to oxygen bonding, epoxy groups, glycol or glycerol, polyhydric alcohol substituents or carbinols, i.e.
Si-CH2-OH
C08G 77/045 takes precedence over this group.
This place covers:
HO-PDMS-OH or condensed siloxane resins of the form RSiOxOHy having Si-OH groups.
This place covers:
RO-PDMS-OR or condensed siloxane resins of the form RSiOxORy having Si-OR alkoxy groups.
This place covers:
Polysiloxanes containing silicon bound to unsaturated aliphatic groups, e.g. vinyl or (meth)acrylate.
C08G 77/045 takes precedence over this group.
This place covers:
Polysiloxanes containing silicon bound to organic groups containing atoms other than carbon, hydrogen and oxygen, e.g. isocyanates or oximes
This place covers:
Physical post-polymerisation treatments which result in no change in length of polysiloxane backbone
This place does not cover:
Chemical after-treatment |
C08G 77/045 takes precedence over this group.
This place covers:
Polysiloxanes modified by chemical after-treatment which result in no change in length of polysiloxane backbone, but in polysiloxanes having substituents to be specified in sub-groups
C08G 77/045 takes precedence over this group.
This place covers:
Preparation of block- or graft-polymers starting from a pre-existing polysiloxane backbone.
This place does not cover:
Polymerising aliphatic unsaturated monomers on to a polysiloxane |
This place covers:
Preparation of block- or graft-polymers containing only polysiloxane sequences, e.g. from a MQ siloxane resin cocondensed with a D siloxane polymer.
This place covers:
Preparation of block- or graft-polymers containing polyurethane sequences, e.g. urethane-urea type copolymers.
This place covers:
Polymers where there is a Si atom in the main chain in which at least two but not all the silicon atoms are connected by carbon linkages, e.g. vinyl endblocked PDMS is reacted with Si-H endblocked PDMS in a stochiometric ratio of >1:1 so that defined macromolecular species are build:
2 ViMDxMVi + HMDxMH → ViMDxMCH2-CH2MDxMCH2-CH2MDxMVi
or the analog reaction scheme with α,ω vinyl endcapped aliphatic hydrocarbons:
ViRVi + HMDxMH → ViR CH2-CH2MDxMCH2-CH2RVi with R being an aliphatic hydrocarbon
C08G 77/485 takes precedence over this group.
This place covers:
Polymers where there is a Si atom in the main chain in which at least two but not all the silicon atoms are connected by carbon linkages containing aromatic rings, e.g.
ViRVi + HMDxMH → ViR CH2-CH2MDxMCH2-CH2RVi with R being an aromatic hydrocarbon.
This place covers:
Polymers where there is a Si atom in the main chain in which at least two but not all the silicon atoms are connected by metal-containing linkages, e.g. silane co-condensation with Ti, Al or Zr alkoxides
C08G 77/485 takes precedence over this group.
This place covers:
Polymers where there is a Si atom in the main chain in which all the silicon atoms are connected by linkages other than oxygen atoms, e.g. polysilanes or polysilcarbenes
This place covers:
Polymers where there is a Si atom in the main chain in which all the silicon atoms are connected by nitrogen atoms, e.g. polysilazanes
This place covers:
Polyphosphazenes are with the repeat unit
-(-RR'P=N-)-n
This place covers:
Polyphosphates
This place covers:
Block copolymers obtained by inter-reacting at least two preformed polymers in the absence of monomers.
Relationship between C08G 81/00 and C08F 299/00
C08F 299/00 refers to macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions.
C08G 81/00 refers to macromolecular compounds obtained by inter-reacting polymers involving reactions other than carbon-to-carbon unsaturated bond reactions.
This place does not cover:
Crosslinking of polymers, i.e. crosslinked macromolecular products obtained by inter-reacting two polymers | |
Intercrosslinking of at least two polymers |
Examples of places where the subject matter of this place is covered when specially adapted, used for a particular purpose, or incorporated in a larger system:
Block or graft polymers obtained by polymerising compounds having carbon-to-carbon double bonds on to polymers | |
Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers; Coatings or adhesives compositions thereof | |
Compositions of block copolymers containing at least one sequence of a polymer obtained by reactions only involving carbon-to-carbon unsaturated bonds;Compositions of derivatives of such polymers; Coatings or adhesives compositions thereof | |
Compositions of homopolymers or copolymers, obtained by polymerisation reactions only involving carbon-to-carbon unsaturated bonds, not provided for in groups C08L 23/00 - C08L 53/00 |
Attention is drawn to the following places, which may be of interest for search:
Polyester-amides | |
Polyamides-imides | |
Polyester-imides | |
Block- or graft polymers containing polysiloxane sequences | |
Involving only carbon-to-carbon unsaturated bond reactions |
This place covers:
Block or graft copolymers obtained by coupling polymers containing sequences of conjugated dienes and of polymers containing vinyl aromatic monomers, e.g. SBR.
Attention is drawn to the following places, which may be of interest for search:
Block or graft polymers containing sequences of polymers of C08C or C08F and of polymers of C08G | |
Macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions, in the absence of non-macromolecular monomers from polysiloxanes | |
Compositions of graft copolymers | |
Compositions of block copolymers | |
Compositions of unspecific macromolecular , obtained otherwise than by polymerisation reactions only involving unsaturated carbon-to-carbon bonds |
Attention is drawn to the following places, which may be of interest for search:
Macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions, in the absence of non-macromolecular monomers from polysiloxanes | |
Compositions of unspecific macromolecular compounds, obtained otherwise than by polymerisation reactions only involving unsaturated carbon-to-carbon bonds |
This place covers:
Attention is drawn to the following places, which may be of interest for search:
Macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions, in the absence of non-macromolecular monomers from polyurethanes |
This place covers:
Attention is drawn to the following places, which may be of interest for search:
Macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions, in the absence of non-macromolecular monomers from unsaturated polyesters | |
Macromolecular compounds obtained by inter-reacting polymers involving only carbon-to-carbon unsaturated bond reactions, in the absence of non-macromolecular monomers from polyurethanes |
This place covers:
This place covers:
Macromolecular compounds containing organic and inorganic sequences, e.g. organic polymers grafted onto silica.
Attention is drawn to the following places, which may be of interest for search:
Compositions of graft polymers in which the polymer, which is obtained by reactions only involving carbon-to-carbon unsaturated bonds, is grafted on to inorganic particles. | |
Coating compositions based on compositions of graft polymers of C08L 51/10. | |
Adhesive compositions based on compositions of graft polymers of C08L 51/10. |
This place covers:
all types of dendritic polymers not classified already in C08G 83/003 and C08G 83/005, such as
i) linear dendritic polymers
ii) dendrigraft polymers
iii) star-hyperbranched polymers
iv) hypergraft polymers.
Attention is drawn to the following places, which may be of interest for search:
(see corresponding note under C08G 83/003 or C08G 83/005):
Medicinal preparations in nanocapsules made of organic macromolecular compounds; dendrimers | |
Medicinal preparations characterised by the non-active ingredients used, where the non-active ingredient is chemically bound to the active ingredient; starburst conjugates, dendrimers or cascade conjugates. | |
Preparations for testing in vivo; nuclear magnetic resonance (NMR) characterised by the carrier; debdrimers, dendrons, hyperbranched compounds | |
Catalysts containing polymer immobilised coordination complexes; e.g. PEG or dendrimer, i.e. molecular weight enlarged complexes | |
Compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules |
Dendritic polymers are materials with a highly branched structure. Dendritic polymers are characterised by structure and not by chemical nature. Since this particular technical field is growing rapidly, and the classification scheme cannot keep its pace with the developments, in the absence of a more suitable place, all types of dendritic polymers are classified in C08G 83/002 or in one of the subgroups.
In patent documents, the following words/expressions are often used as synonyms:
- "Dendrigraft" and "dendritic polymers"
- "Hypergraft" and "idem"
This place covers:
Dendrimers; i.e. polymers having a core from which emanates an exponentially increasing number of dendritic branches.
Attention is drawn to the following places, which may be of interest for search:
Medicinal preparations in nanocapsules made of organic macromolecular compounds; dendrimers | |
Use of antigens or antibodies in immunisation | |
Medicinal preparations characterised by the non-active ingredients used, where the non-active ingredient is chemically bound to the active ingredient; starburst conjugates, dendrimers or cascade conjugates. | |
Preparations for testing in vivo; nuclear magnetic resonance (NMR) characterised by the carrier; debdrimers, dendrons, hyperbranched compounds | |
Catalysts containing dendrimers | |
Catalysts containing polymer immobilised coordination complexes; e.g. PEG or dendrimer, i.e. molecular weight enlarged complexes | |
Compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Dendrimers |
See corresponding note under C08G 83/002.
In patent documents, the following words/expressions are often used as synonyms:
- "Starburst" and "dendrimer"
- "Arborol(s)", "Dendritic polymer(s)", "Dendron(s)" and "idem"
This place covers:
Hyperbranched polymers; i.e. polymers having a tree-like structure.
Attention is drawn to the following places, which may be of interest for search:
Compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules | |
Coating compositions of unspecified macromolecular compounds containing dendritic macromolecules |
See corresponding note under C08G 83/002.
This place covers:
Polyrotaxanes; polycatenanes.
Attention is drawn to the following places, which may be of interest for search:
Cosmetic or similar toilet preparations containing cyclodextrins | |
Compositions of polyalkylene oxides | |
Coating compositions of polyalkylene oxides | |
Adhesive compositions of polyalkylene oxides |
A polyrotaxane comprises a linear molecule and cyclic molecules in which the linear molecule is included in cavities of cyclic molecules in a skewered manner, and capping groups, each of which locates at each end of the linear molecule in order to prevent the dissociation of the cyclic molecule.
In patent documents, the following words/expressions are often used as synonyms:
- "Rotaxane(s)" and "polyrotaxane(s)" or "polycatenane(s)"
- "Catenane(s)", "Cyclodextrin(s)", "Crown ether(s)" and "idem"
This place covers:
Supramolecular polymers, where the monomer units are held together by reversible secondary interactions in the main chain.
Supramolecular polymers are polymeric arrays of monomer units, held together by reversible and highly directional secondary interacting -that is, non-covalent bonds, such as hydrogen bonds.
In patent documents, the following words/expressions are often used as synonyms:
- "Self-assembling polymer(s)" and "supramolecular polymer(s)"
C08F is referred to in this group because there is no group under C08F corresponding to C08G 85/00. So although it would in principle not appear correct to classify here post- or after-treatment of such polymers (C08F), which have not already been classified elsewhere, a place must in practice be found here for such documents.
This place covers:
Attention is drawn to the following places, which may be of interest for search:
After treatment of condensation or polyaddition polymers | C08G 2/30, C08G 59/14, C08G 63/46, C08G 63/91, C08G 64/42, C08G 65/32, C08G 65/48, C08G 69/48, C08G 75/0286, C08G 77/38, C08G 85/004 |
Chemical modification of membranes | |
After treatment of addition polymers, e.g. obtained by reactions involving polymers obtained by reactions involving carbon to carbon unsaturated bonds or purification | |
Recovery or working-up of waste polymers |
This place covers:
preventing the deposition of scale in polymerisation reactors.
Attention is drawn to the following places, which may be of interest for search:
Scale prevention in a polymerisation reactor or its auxiliary parts |
This place covers:
Cleaning reaction vessels with chemicals, e.g. polymerisation reactors or extruders.
Attention is drawn to the following places, which may be of interest for search:
Mechanical methods | |
Cleaning extruder parts |